
Divide the solution by 100 and add the findings if isotope abundance is present. Each isotope’s abundance is multiplied by its mass. A list of isotopes with natural abundance and mass is given a percentage or decimal value. These recommendations can be used to calculate the atomic mass of the component. Weighted Average for All Atoms of an Element – The weighted average of each element’s isotopes, grounded in nature by their abundance, is the atomic weight of that element.Adding the Mass of Protons and Neutrons – The total number of protons and neutrons in an atomic nucleus gives the mass number.By looking up to the atomic mass on the periodic table- In the periodic table digit of an atomic mass is usually marked under the representation of an element.Here are three ways to determine the Atomic Mass, depending on the given conditions:
#Atomic mass definition how to
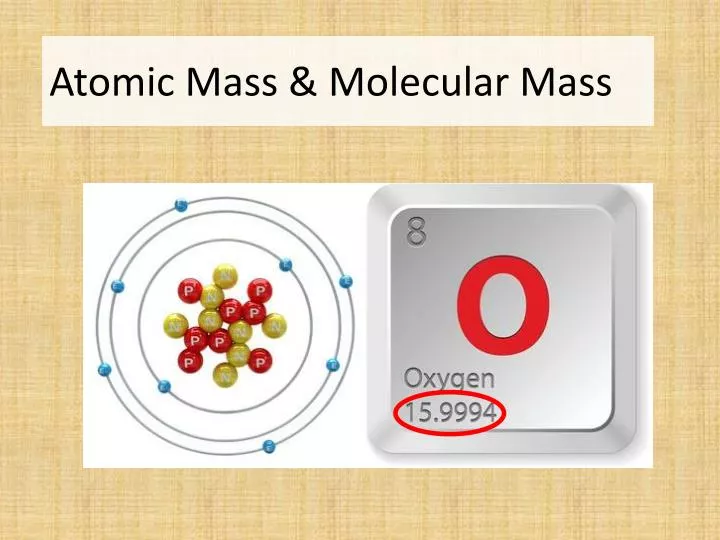
Mass Number = Number of Protons + Number of Neutrons How to calculate the Atomic Mass?

The formula below can be used to determine the mass number of an atom of a specific element when looking at the periodic table of elements: While the atomic mass or atomic weight is the average number of protons and neutrons in an element for all of its isotopes.

The difference between the mass number and the atomic mass of an element is that a mass number is a whole number obtained by the addition of a number of protons and neutrons. ISRO CS Syllabus for Scientist/Engineer Exam.ISRO CS Original Papers and Official Keys.GATE CS Original Papers and Official Keys.


 0 kommentar(er)
0 kommentar(er)
